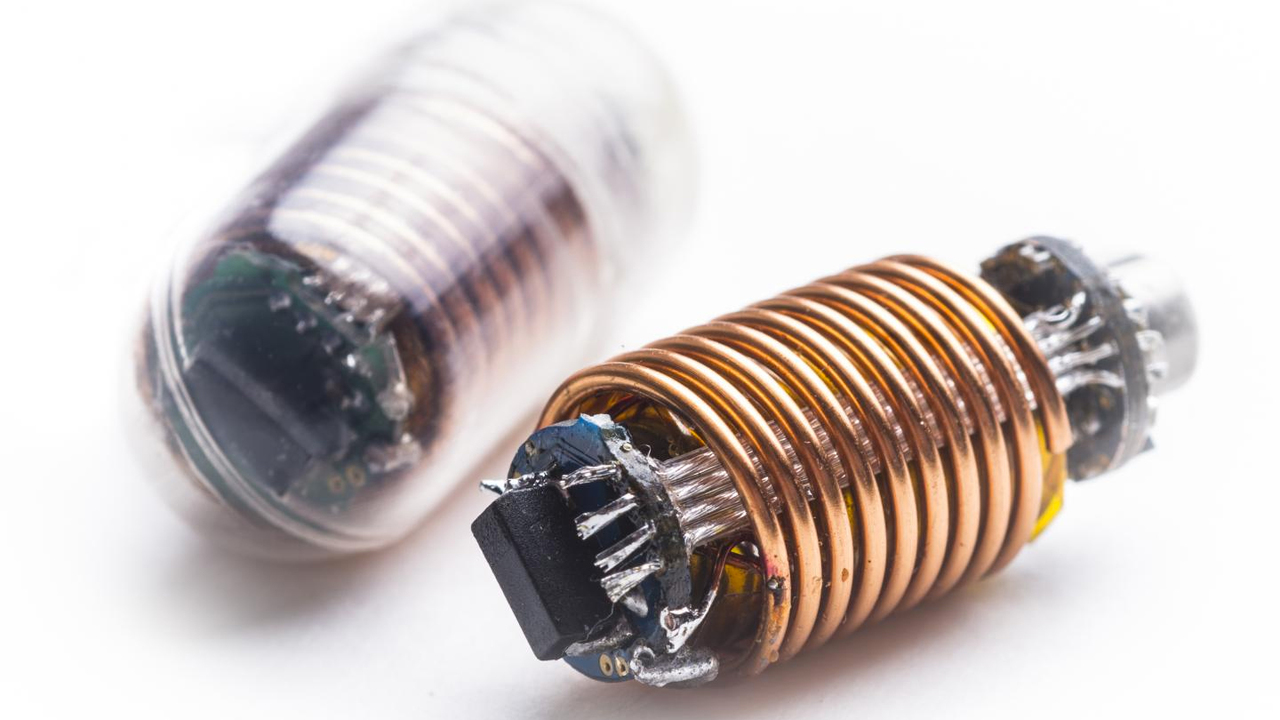
The US Food and Drug Administration (FDA) approved for the first time in history the sale of an electronic tablet with a built-in microchip. The "smart" pill has a tiny sensor that allows you to determine whether the patient adheres to the prescribed course of taking medication.
Novelty - Abilify MyCite - was developed by the medical company Otsuka Pharmaceutical in collaboration with Proteus Digital Health. It is a type of Abilify, Otsuka, which treats depression, bipolar disorder and schizophrenia. A smart tablet that activates under the influence of liquids in the stomach reminds the patient if he forgot to take regular medications, studies the reaction of the body to them, monitors the movements of a person and sends all this information to a mobile phone.
Abilify MyCite passes through the gastrointestinal tract and leaves the body naturally in about a day. After one cycle, the "robocopil" can be cleaned and used again.
According to the representative of the FDA Mitchell Mathis, the development of Otsuka and Proteus can be very effective, especially when it comes to adherence to people with mental disabilities. At the same time, "smart" tablets cause a lot of questions related to privacy. They are essentially a microchip in the shell, so they are probably somehow susceptible to breaking. So, third parties, including insurance companies, can easily learn about the state of your health.
© All rights reserved. Citing to www.ict.az is necessary upon using news





